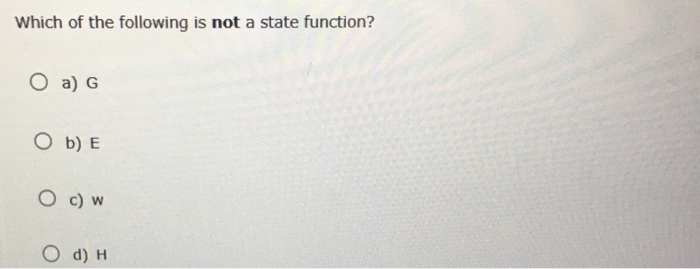
Which Of The Following Is Not A State Function . The same can be said for the volume, pressure, and the number of moles of gas in the sample. Maharashtra state board previous year question paper with solution for class 12 science;
নিচের কোনটি রাষ্ট্রীয় কাজ নয়? (ই)Q+W (দ্বিতীয়)Q (Iii)W (চতুর্থ)H-Ts from www.doubtnut.com
Of the following, only _____ is not a state function. Heat (q) and work (w) are not state functions being path dependent.
নিচের কোনটি রাষ্ট্রীয় কাজ নয়? (ই)Q+W (দ্বিতীয়)Q (Iii)W (চতুর্থ)H-Ts
P 1v 1 = p 2v 2. A state function is the property of the system whose value depends only on the initial and final state of the system and is independent of the path. Nvl is a general function used to provide alternate value to the null values.the functions max, min and avg can be used as group by functions.
Source: www.chegg.com
Maharashtra state board previous year question paper with solution for class 12 arts; This will be automatically true for any valid mathematical function since this is a condition on functions from mathematical analysis. No matter how many times we heat, cool, expand, compress, or otherwise change the system, the net change in the temperature only depends on the initial and.
Source: slideplayer.com
No matter how many times we heat, cool, expand, compress, or otherwise change the system, the net change in the temperature only depends on the initial and final states of the system. A state function is simply one that depends only on the start and end point, and not the path. So, it is a state function.
Source: www.toppr.com
The same can be said for the volume, pressure, and the number of moles of gas in the sample. Nvl is a general function used to provide alternate value to the null values.the functions max, min and avg can be used as group by functions. Select the most appropriate option.
Source: www.chegg.com
Of the following, only _____ is not a state function. It is also extensive property. This will be automatically true for any valid mathematical function since this is a condition on functions from mathematical analysis.
Source: www.chegg.com
Hence, the correct option is b. So, it is a state function. The same can be said for the volume, pressure, and the number of moles of gas in the sample.
Source: www.chegg.com
Which of the following is not a state function? Hence, the correct option is b. Experts are tested by chegg as specialists in their subject area.
Source: www.toppr.com
Heat volume altitude internal energy. State functions can be considered as integrals. Of the following, only _____ is not a state function.
Source: www.chegg.com
Maharashtra state board previous year question paper with solution for class 10 P 1v 1 = p 2v 2. It does not depend on how we got from initial to final state.
Source: www.transtutors.com
A state function is the property of the system whose value depends only on the initial and final state of the system and is independent of the path. In the same way, one does not concern his or her self with the amount of “heat” that an object has, since it is merely a term used to denote the amount.
Source: www.toppr.com
Moreover, we say that this potential energy is a state function because it depends only on the initial and final heights of the mass in question. No matter how many times we heat, cool, expand, compress, or otherwise change the system, the net change in the temperature only depends on the initial and final states of the system. Maharashtra state.
Source: www.youtube.com
Energy (e) enthalpy (h) heat (q) temperature (t) entropy (s) question: (a) 46 m3 (b)1900 m3 (c) 0.022 m3 (d) 54 m? In the same way, one does not concern his or her self with the amount of “heat” that an object has, since it is merely a term used to denote the amount of transferred energy between systems as.
Source: www.clutchprep.com
A) delta h = 0 b) delta s = 0 c) delta h = p delta v d) delta h = delta nrt e) delta h = t delta s. Lambda includes a state field in the function configuration for all functions to indicate when your function is ready to invoke. Internal energy, pressure, volume, temperature are all state functions.
Source: www.chegg.com
Function states do not change the behavior of function invocations or how your function runs the code. Which of the following is not a state function? The function, lower limit and its upper limit.
Source: www.doubtnut.com
State provides information about the current status of the function, including whether you can successfully invoke the function. What is the new volume of gas? As you said the wave function must be square integrable.
Source: www.doubtnut.com
This is because, integrals depend on only three things: If you heat 250 g of water from a temperature of 21 degrees celsius to 29 degrees celsius, how much heat is absorbed by the water (in kj)? Experts are tested by chegg as specialists in their subject area.
Source: www.chegg.com
A) delta h = 0 b) delta s = 0 c) delta h = p delta v d) delta h = delta nrt e) delta h = t delta s. The path functions are irreversible expansion work and reversible expansion work. Which of the following are not state functions?
Source: www.chegg.com
This means entropy is a state function. It is also extensive property. Entropy is defined as thermal energy per unit temperature that is available for doing work.
Source: www.toppr.com
(a) concentration (b) internal energy (c) enthalpy A state function depends only on the final and the initial state of the system. This is because, integrals depend on only three things:
Source: www.chegg.com
This is because, integrals depend on only three things: It is a state function because it is independent of the path. Number of particles water has a specific heat of 4.184 j/gk.
Source: www.toppr.com
Experts are tested by chegg as specialists in their subject area. It is also independent of the path (as energy and temperature are path independent). Maharashtra state board previous year question paper with solution for class 12 arts;