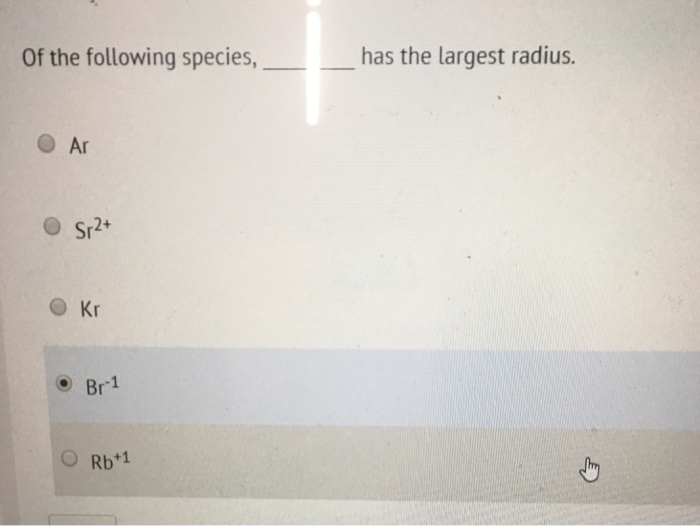
Which Of The Following Has The Largest Radius . Which ion below has the largest radius? (©) 1s?, 2s?, 2p', 3s, 3p3 (d) 1s2, 2s2, 2p\, 3s2, 3ps arrange the elements in increasing order of atomic radius
Solved Which Of The Following Elements Has The Largest | Chegg.com from www.chegg.com
Thus, the order of increasing radius is `n lt c lt p lt si`. Which ion below has the largest radius?
Solved Which Of The Following Elements Has The Largest | Chegg.com
The h nucleus has a single positive charge. Cations are smaller than their parent atoms because of increase in nuclear charge. Similarly, both `si` and `p` are in the third period and `si` is to left of `p`, therefore, teh radius of `p` is smaller than that of `si`.
Source: www.chegg.com
Similarly, both `si` and `p` are in the third period and `si` is to left of `p`, therefore, teh radius of `p` is smaller than that of `si`. Which of the following has the largest radius? Since sulfur is to the left of chlorine on the regular table, the will have a larger atomic radius.
Source: oneclass.com
Which of the following species has the largest radius m | which of the following species has the largest radius? As a result orbital electrons become more closer to nucleus. As you go down the table, more electron shells are added to make the radius larger, but as you go to the right, the protons' attraction.
Source: www.youtube.com
Which of the following atoms has the largest radius? Of the atoms here, the br atom would be the largest as it is farthest down the group and hence its anion also will be the largest ion. Therefore, 1 s 2, 2 s 2, 2 p 6, 3 s 2 has the largest radius.
Source: www.clutchprep.com
Hence, li has the largest atomic radius and f has the smallest atomic radius. A) sr b) ca c) y d) rb e) k A) na b) ba c) ca d) cs.
Source: www.chegg.com
Thus atomic radius is directly proportional to the number of electron shells. So we are going to discuss which of the fallen iron has the highest any radius radius increase. Which atom has the largest negative electron affinity?
Source: www.quora.com
This homework question can be solved by thinking about how the charges react to each other. Hence, li has the largest atomic radius and f has the smallest atomic radius. (©) 1s?, 2s?, 2p', 3s, 3p3 (d) 1s2, 2s2, 2p\, 3s2, 3ps arrange the elements in increasing order of atomic radius
Source: www.numerade.com
A) rb b) si c) s d) o. Therefore, 1 s 2, 2 s 2, 2 p 6, 3 s 2 has the largest radius. Thus ions will have radii different from the atoms because ions will have either gained or lost electrons.
Source: www.chegg.com
This answer is not useful. Think of it this way: Because of the decrease in the effective nuclear charge, any sad part sodium has the largest difference between the first ionization energy on the second ionization energy.
Source: www.youtube.com
Because of the charge present on it. The ionic radii of cations follow the same trends as atomic radii. Which ion below has the largest radius?
Source: www.toppr.com
A) na b) al c) n d) f. 2) what happens to the orbitals, and thus to atoms or ions with a given number of electrons (let us say, 18 electrons), when you increase the atomic. So, the correct answer is option c.
Source: www.toppr.com
Thus atomic radius is directly proportional to the number of electron shells. Which atom has the highest first ionization energy? Magnesium has one more proton than sodium, and therefore, with the same number of electrons and 1s orbital shielding, the zeff for magnesium is greater, and therefore mg2+ has the smaller ionic radius.
Source: www.chegg.com
Magnesium has one more proton than sodium, and therefore, with the same number of electrons and 1s orbital shielding, the zeff for magnesium is greater, and therefore mg2+ has the smaller ionic radius. Atomic size increases with the increase in the number of electron shells. As you go down the table, more electron shells are added to make the radius.
Source: www.chegg.com
Knowing this, we have the right to compare the possible options. Well, i only see two elements out of the four options, but out of those two, cl has a larger atomic radius. Which of the following atoms has the largest radius?
Source: socratic.org
Cations are smaller than their parent atoms because of increase in nuclear charge. The h nucleus has a single positive charge. How do ions compare in size?
Source: www.chegg.com
Magnesium has one more proton than sodium, and therefore, with the same number of electrons and 1s orbital shielding, the zeff for magnesium is greater, and therefore mg2+ has the smaller ionic radius. A) cs b) ca c) al d) o. (©) 1s?, 2s?, 2p', 3s, 3p3 (d) 1s2, 2s2, 2p\, 3s2, 3ps arrange the elements in increasing order of.
Source: www.toppr.com
As a result orbital electrons become more closer to nucleus. A) sr b) ca c) y d) rb e) k Thus, the ion with the largest radius is closest to the lower left corner of the periodic table, and that is the k+ ion.
Source: www.chegg.com
2) what happens to the orbitals, and thus to atoms or ions with a given number of electrons (let us say, 18 electrons), when you increase the atomic. Thus, the order of increasing radius is `n lt c lt p lt si`. As the nuclear charge increases the attractive force between nucleus and electrons increases.