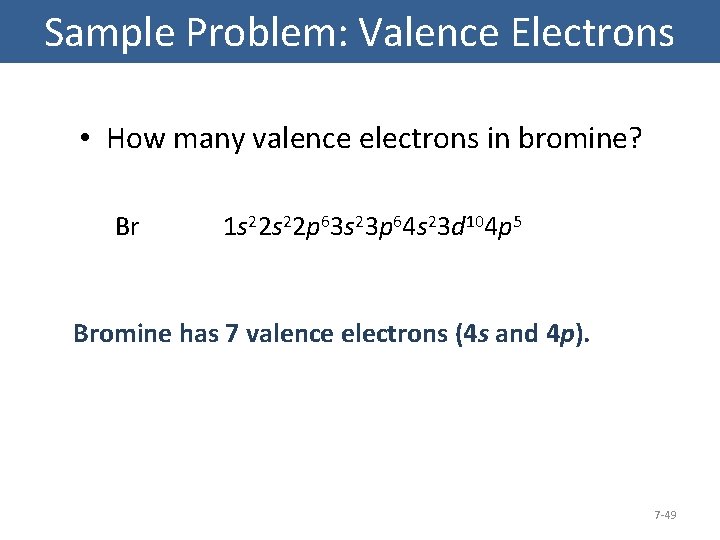
1S22S22P63S23P64S23D104P5 Which Element Is This . Which element has an electron configuration of kr5s2? The electron configuration for cesium is as follows:
Ppt - Chapter 13 Powerpoint Presentation, Free Download - Id:2061143 from www.slideserve.com
Fe2+ 10 in the space below, write the abbreviated electron configurations of the following elements co 10) lr determine what elements are denoted by the following electron configurations: Bromine has 7 electrons in its outermost shell which acts as its valence electrons.
Ppt - Chapter 13 Powerpoint Presentation, Free Download - Id:2061143
Is group 14 metal or nonmetal? Enter the name of the element for the corresponding electron configuration: (a) 1s22s22p63s23p64s23d1 (b) 1s22s22p63s23p64s23d104p65s1 (c) 1s22s22p63s23p64s23d104p5 (d) 1s22s22p63s23p4.
Source: www.numerade.com
Elements that are nonmetals tend to gain electrons and become negatively charged ions called anions. Hence the full or expanded electronic configuration for bromine in accord with the aufbau principle is 1s22s22p63s23p64s23d104p5. This element is bromine (br).
Source: brainly.com
What is the formula of the ion. Sr has the above electron configuration. (2) the element with an electron configuration of 1s22s22p63s23p64s23d104p5 is in group fill in the blank 3 and period.
Source: brainly.com
Elements that are nonmetals tend to gain electrons and become negatively charged ions called anions. The electron configuration 1s^2 2s^2 2p^6 3s^2 3p^2 is the element silicon. The electron configurations in this worksheet assume that lanthanum (la) is the first element in the 4f block and that actinium (ac) is the first element in the 5f block.
Source: slideplayer.com
Defining what an element is? What is mendeleev known for? Which phase is represented by the yellow area?
Source: www.clutchprep.com
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6. Those are the small number placed at the top after the letters. Its the same as the elements outermost electron shell number.
Source: slideplayer.com
1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6. Those are the small number placed at the top after the letters. Sr has the above electron configuration.
Source: slideplayer.com
Which phase is represented by the yellow area? (a) 1s22s22p63s23p64s23d1 (b) 1s22s22p63s23p64s23d104p65s1(c) 1s22s22p63s23p64s23d104p5 (d) 1s22s22p63s23p4 1s22s22p63s23p64s23d104p5 1s22s22p63s23p64s23d104p6 1s22s22p63s23p44s23d104p5 1s22s22p63s23p64s23d94p5 2 see answers alleei alleei answer.
Source: www.clutchprep.com
What is the electron configuration for aluminum? How many valence electrons are in the following 1s22s22p63s23p64s23d104p5? What is the significance of the period or row number of an element on the periodic chart?
Source: slideplayer.com
Which element has an electron configuration of kr5s2? Solution for identify the elements two of these are irregular so pay close attention. (1) electronic configuration of the element:
Source: www.chegg.com
Which element has the following electron configuration? Experts are tested by chegg as specialists in their subject area. The atomic number of bromine is 35, which means it has 7 electrons in its valence shell.
Source: www.slideshare.net
1s2, 2s2 2p6, 3s2 element classification: 1s2 2s2 2p6 3s2 3p6 4s2 3d6 number of electrons in the element = 2+2+6+2+6+2+6 = 26 the atomic number of the element = number of protons in the element = number of. What is the electron configuration for aluminum?
Source: www.slideserve.com
(a) 1s22s22p63s23p64s23d1 (b) 1s22s22p63s23p64s23d104p65s1 (c) 1s22s22p63s23p64s23d104p5 (d) 1s22s22p63s23p4. Sir humphrey davy discovery date: (1) electronic configuration of the element:
Source: www.chegg.com
Of valence shell, i.e., 5group no. This element is bromine (br). In the space below, write the unabbreviated electron configurations of the following elements.
Source:
Sr has the above electron configuration. The atomic number of bromine is 35, which means it has 7 electrons in its valence shell. The electron configuration of bromine is 1s22s22p63s23p64s23d104p5 and the outer most shells are the 4s and 4p orbitals.
Source: brainly.com
Why does the 3d sublevel fill before the 4p sublevel in the electron configuration for bromine? 1s22s22p63s23p64s23d104p5 1s22s22p63s23p64s23d104p6 1s22s22p63s23p44s23d104p5 1s22s22p63s23p64s23d94p5 2 see answers alleei alleei answer. Its the same as the elements outermost electron shell number.
Source: studylib.net
1s2 2s2 2p6 3s2 3p6 4s2 3d6 number of electrons in the element = 2+2+6+2+6+2+6 = 26 the atomic number of the element = number of protons in the element = number of. 1s22s22p63s23p64s23d104p5 1s22s22p63s23p64s23d104p6 1s22s22p63s23p44s23d104p5 1s22s22p63s23p64s23d94p5 2 see answers alleei alleei answer. (a) 1s22s22p63s23p64s23d1 (b) 1s22s22p63s23p64s23d104p65s1(c) 1s22s22p63s23p64s23d104p5 (d) 1s22s22p63s23p4
Source: slidetodoc.com
The electron configuration 1s^2 2s^2 2p^6 3s^2 3p^2 is the element silicon. The electron configuration for cesium is as follows: Those are the small number placed at the top after the letters.
Source: www.chegg.com
Atomic no.=z= 47elctronic configuration= 1s 22s 22p 63s 23p 64s 23d 104p 65s 24d 9the period is the no. (a) 1s22s22p63s23p64s23d1 (b) 1s22s22p63s23p64s23d104p65s1 (c) 1s22s22p63s23p64s23d104p5 (d) 1s22s22p63s23p4. Carbon group element, any of the six chemical elements that make up group 14 (iva) of the periodic table—namely, carbon (c), silicon (si), germanium (ge), tin (sn), lead (pb), and flerovium (fl).
Source: slideplayer.com
(a) 1s22s22p63s23p64s23d1 (b) 1s22s22p63s23p64s23d104p65s1 (c) 1s22s22p63s23p64s23d104p5 (d) 1s22s22p63s23p4. Experts are tested by chegg as specialists in their subject area. The electron configuration 1s^2 2s^2 2p^6 3s^2 3p^2 is the element silicon.
Source: slidetodoc.com
Elements that are metals tend to lose electrons and become positively charged ions called cations. Elements that are nonmetals tend to gain electrons and become negatively charged ions called anions. 1s22s22p63s23p64s23d104p5 which element is this?.